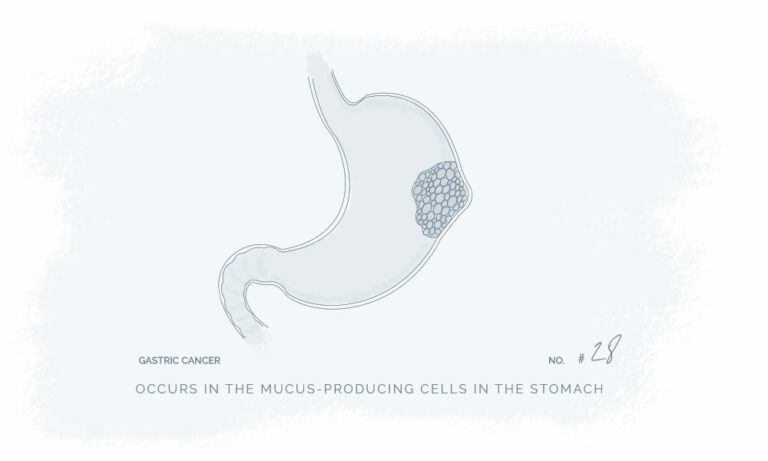
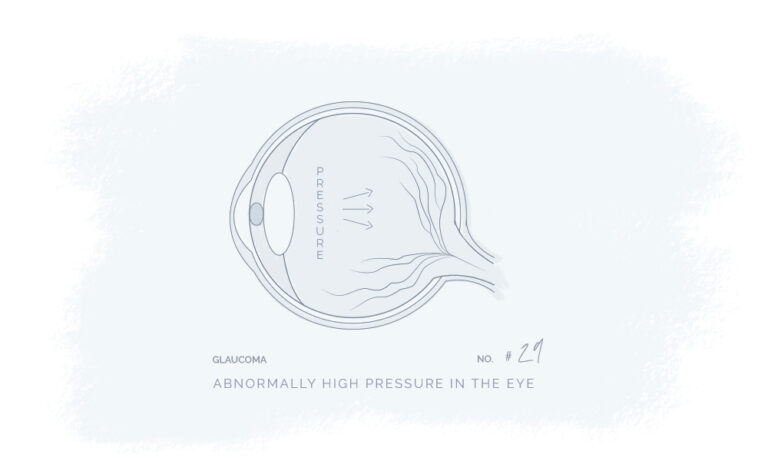
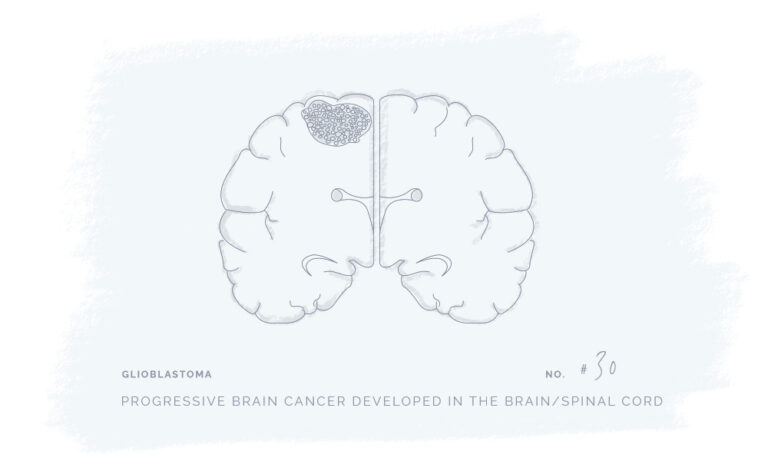
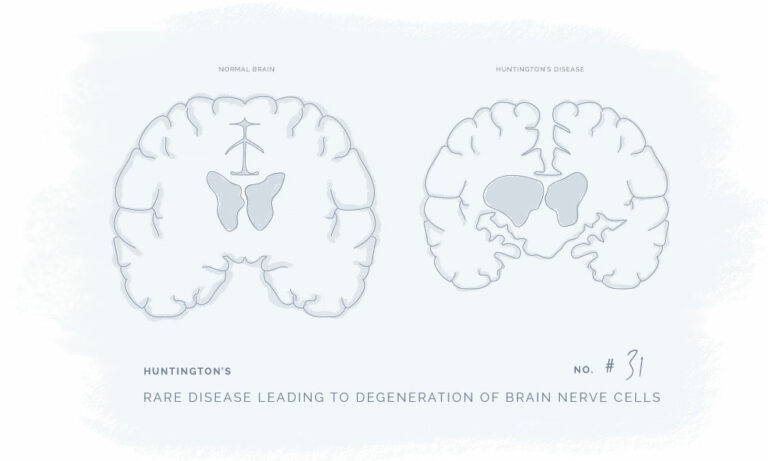
Natural Products for a Better Every Day
Clinically documented innovation powered by patented CBD technology
Our overarching purpose is increasing the life quality of patients dealing with autoimmune and stress-related disorders worldwide.
We provide our customers with effective, natural and safe treatments that help them recover their bodies natural balance.
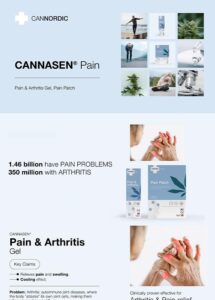
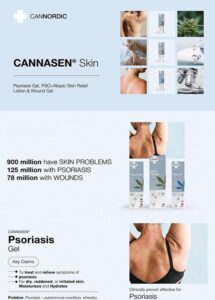
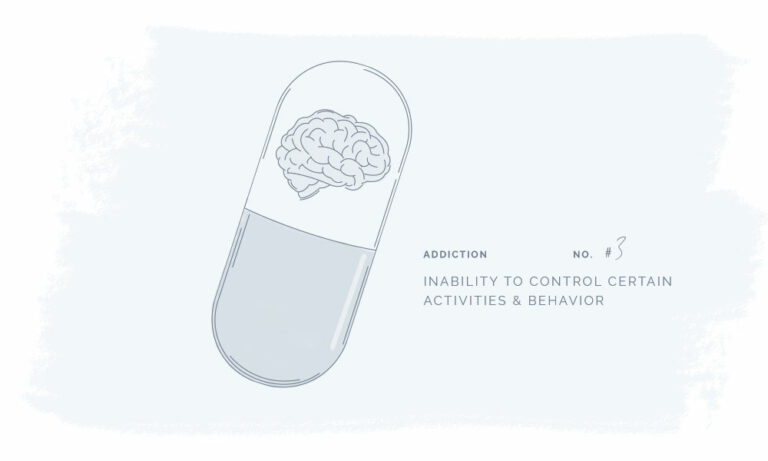
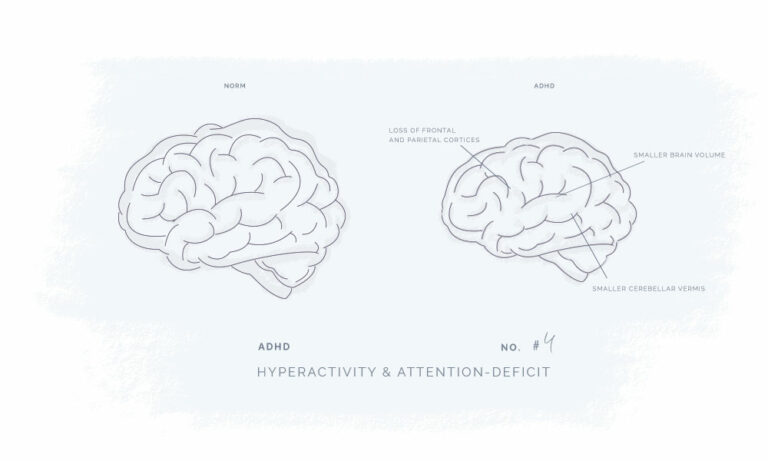
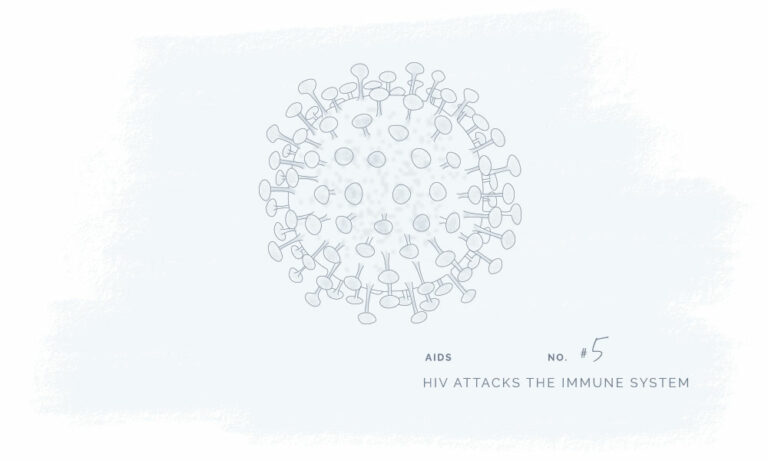
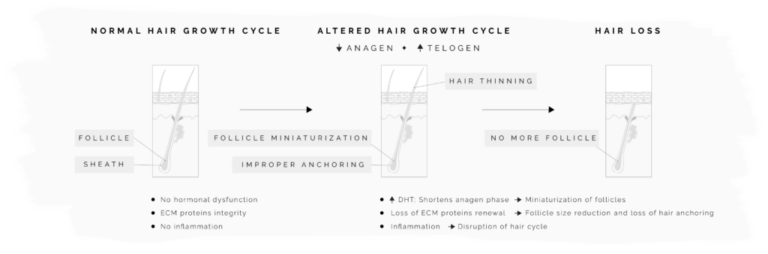
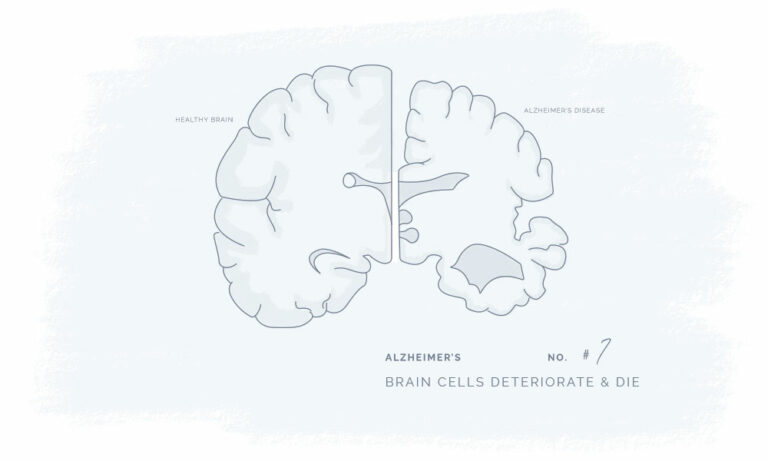
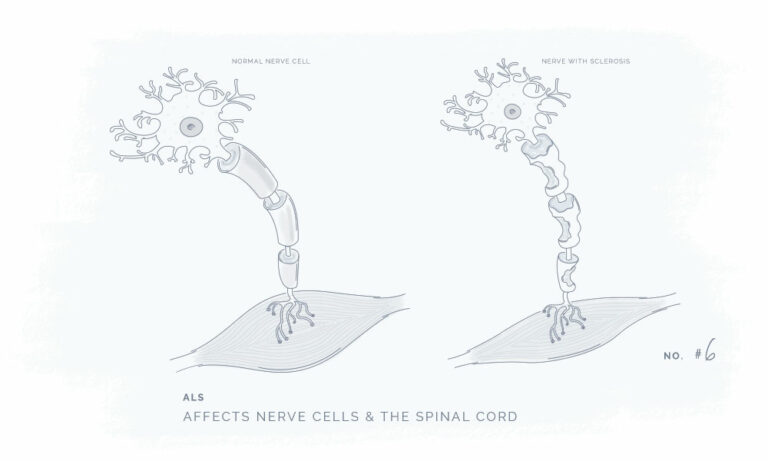
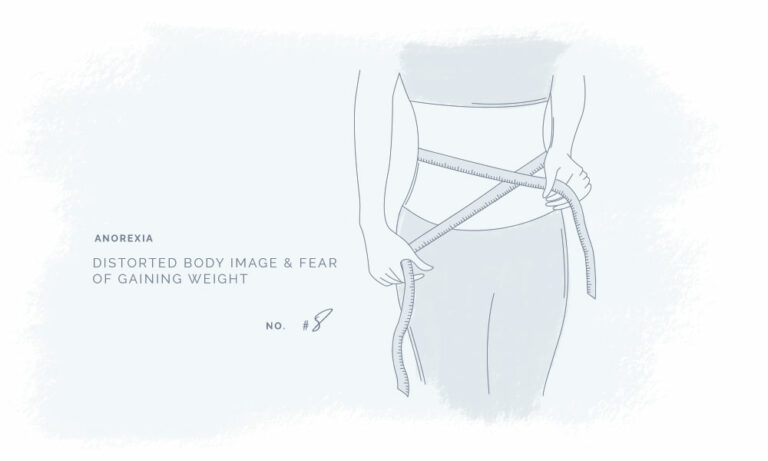
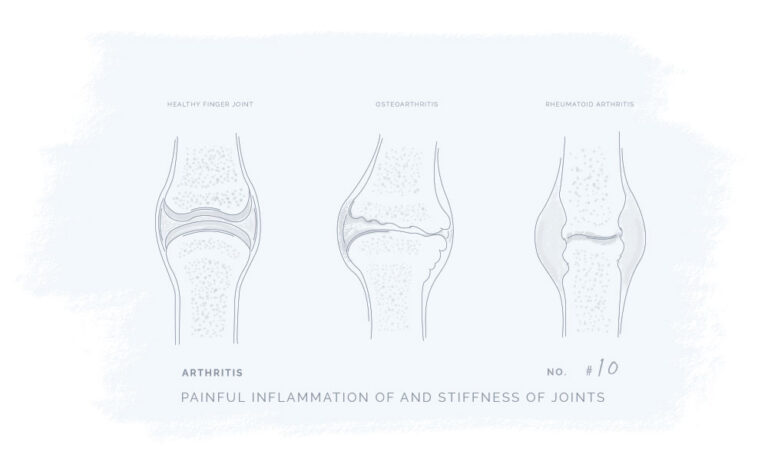
All our products have been meticulously developed to treat and relieve pain, arthritis, skin problems, allergy, sleep disorders, hair-loss and animal treatment.
Medical Device
All CANNASEN® treatment products are registered as medical devices and are available OTC
First Mover Company
We develop, manufacture and sell registered medical devices with cannabinoids as the first in the market.
Efficient OTC with CBD
CANNASEN® products have documented efficacy and safety, and are the alternative to conventional medical treatment.
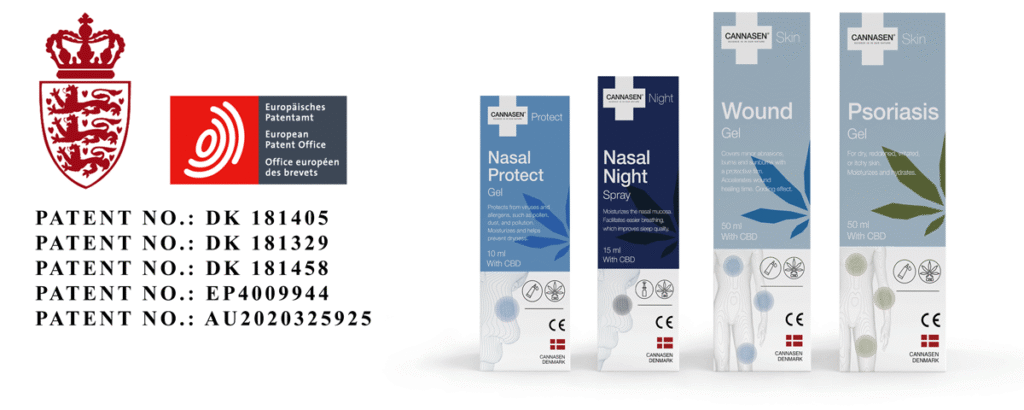
CE Marked and ISO 13485
CANNASEN® products are CE and ISO 13485 marked to ensure compliance with legislation and quality assurance.
Highest Quality CBD
We use high quality crystalized CBD originated from hemp in all CANNASEN® products
Patents Pending
A patent is pending for all CANNASEN® treatment products.